
Why ERP still feels like a “dread project” in life sciences
Up to 75% of large transformation programs fail to deliver their stated objectives, and ERP is consistently on the podium of disappointment. In GxP‑regulated biotech, pharma and medtech, the irony is sharp: companies invest millions in life sciences ERP systems to increase control, yet teams on the ground experience more spreadsheets, more firefighting and very little operational resilience.
This article is written for doers: CFOs, COOs, Heads of Quality, Supply Chain and Digital who sit in the middle of that tension. The goal is simple: show how to turn ERP from a Finance/IT upgrade into a value engine, using a concrete 5‑step framework you can put in front of a Board in your next meeting.
In this episode of The ERP Perspective, Rui Teixeira CEO of LifeScience Services, we discuss the C-Suite mindset shift and how to turn the ERP into a Value Engine in Life Sciences (Instead of a Dread Project).

Step 1 – Make Phase 0 a board‑level governance decision
What is Life Sciences ERP systems migration readiness?
Most mid‑tier life sciences companies skip Phase 0 or compress it into vendor demos and a price comparison. In practice, that is where ERP failure is baked in.
For biotech scale‑ups and pharma manufacturers, Phase 0 is not a consulting exercise; it is a governance decision where the Board must:
- Declare ERP a company milestone, explicitly tied to strategic goals such as EU launch, tech transfer or CDMO onboarding.
- Assign RACI and OKRs for senior leaders so ERP sponsorship becomes part of their performance review, not “optional work”.
- Set the emotional tone: raising issues early is rewarded, not punished — especially around GxP, CSV and supply chain risk in biotech.
In return, the CFO and program leads should bring back three concrete outputs:
- A high‑level solution blueprint (for example, SAP S/4HANA for biotech plus validated cloud ERP components) that fits the current and future operating model.
- A readiness check across data quality, process maturity and culture, including GAMP 5 system validation gaps and Annex 11 expectations.
- Two to three quick wins that free cash or reduce risk before the big program even starts. Start with archiving, data cleanup, or simple automation that will solve actual pain points.
Skipping this looks faster but is equivalent to borrowing time at a very high interest rate in scope creep, rework and lost trust.
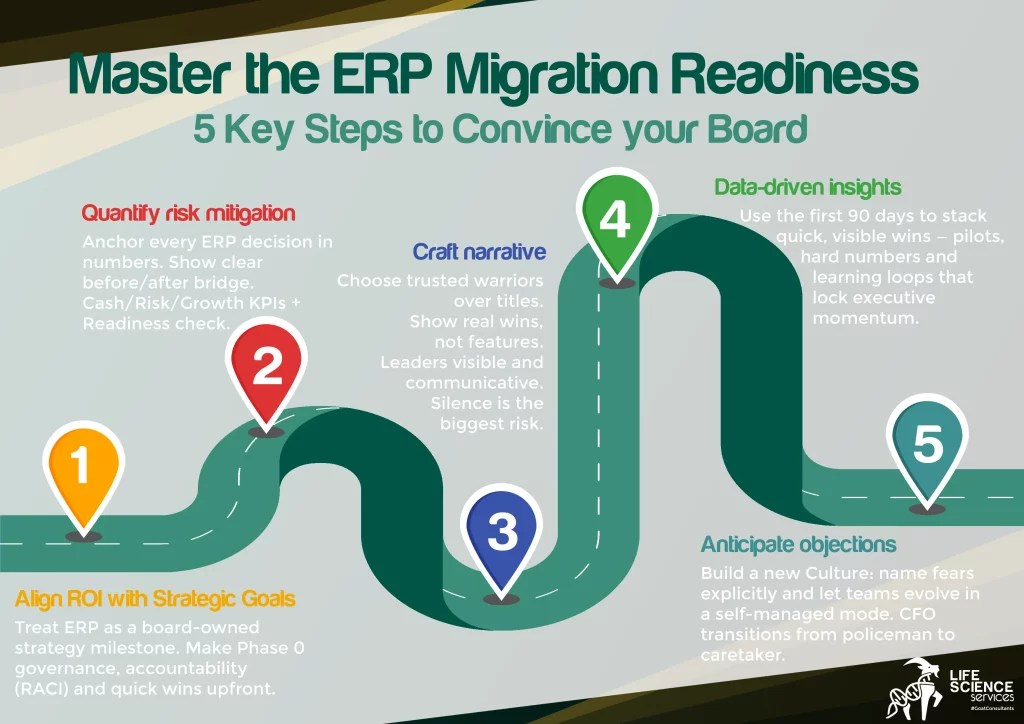
Life Sciences ERP systems migration readiness roadmap
ERP migration isn’t an IT sprint; it’s a staged operating-model shift.
This roadmap visualises how life sciences ERP systems evolve from chaos to controlled, GxP-compliant, scalable operations across biotech, pharma and medtech networks.
Research confirms the problem. Studies of organization design show that rigid structures often create “misfits” between internal processes and external demands . These misfits reduce performance, hinder innovation, and ultimately disengage employees.
Step 2 – Anchor everything in three words: Cash, Risk, Growth
Boards don’t wake up caring about modules or configurations. They care about runway, downside and scalability. In practice, every life sciences ERP decision can and should be framed around three outcomes: cash, risk and growth.
- Cash: For VC‑backed biotech, a “large” ERP budget often buys only 2–3 years of runway. A six‑month delay in a critical R&D stream or tech transfer can be lethal. ERP must show how it moves DSO, inventory days, past‑due receivables and close cycle — turning cash into a dial you can actually turn.
- Risk: Many medicine shortages and quality deviations trace back to internal manufacturing, documentation and supply chain failures. A GxP‑compliant supply chain depends on BOMs linked to SOPs, real‑time maintenance and demand signals connected to production. That is exactly where life sciences ERP systems either shine or fail.
- Growth: Scaling biotech operations, adding new markets or onboarding a CDMO should not require doubling the finance and QA headcount. ERP is the operating system that harmonises processes, supports computer system validation (CSV) and keeps compliance automation GxP‑ready while revenue grows.
Board packs for Life Sciences ERP systems migration readiness, the pattern is always the same:
- Quantify today’s pain in their language (DSO, audit findings, time‑to‑release, stock‑outs).
- Show how ERP changes those numbers with a simple before/after bridge.
- Protect the payback and runway they actually care about.
Step 3 – Upgrade the culture: champions, not princes
In most failed ERP migrations, the root cause is not bad software; it is culture. People are afraid, overloaded or invisible in the story.
Three moves make a disproportionate difference:
- Choose warriors, not princes. Many pharma digital transformation efforts name the most senior title as “key user”. In reality, you need the person on the shop floor whom others already follow: the colleague who understands batch records, or the planner who keeps the cold chain intact. Their trust is worth more than any steering committee slide.
- Communicate with real problems, not feature tours. Early demos should not showcase 200 fields and features. It should solve one painful issue, such as reconciling serialized stock or cleaning up deviations in a quality system integration. When people see one source of friction disappear, the narrative shifts from scepticism to curiosity.
- Make leadership visibly present and safe. If the COO never attends working sessions, or the Head of Quality only shows up to escalate, teams conclude that ERP is a technical project and will play it safe. Leaders who regularly show up, ask what scares people, and explicitly state that errors in pilots are learning data, not grounds for blame, remove the biggest psychological blocker: silence.
This is where medtech team engagement becomes a real metric: are the people who live inside CAPAs, change controls and supply chain disruption actually in the room?
SAP featured LifeScience Services as a success story in ERP transformation. This case shows how we delivered a high-stakes SAP Business ByDesign migration in a complex, regulated Biotech environment.
Step 4 – Use the first 90 days to prove it works
For operational resilience in biotech and pharma, the emotional tone of the program is set in the first 90 days. That is when teams decide whether ERP is another top‑down burden or a tool that gives them time and clarity back.
A practical pattern:
Days 1–30 – Build conviction.
Set up a small cross‑functional “Champions’ team” with Finance, QA, Supply Chain, Operations and IT/digital transformation. Ask each function for its single biggest pain point: unreconciled inventory, repeated CSV documentation, late vendor payments, etc. Identify the most sceptical person in the room; make them your reference success story.
Days 30–60 – Show real progress.
Launch 1–2 low‑risk pilots, such as a basic order‑to‑cash flow with serialization, or a single warehouse with improved inventory accuracy. Show concrete improvements: fewer manual entries, cleaner GxP audit trails, faster reconciliation of controlled substances or cold chain deviations. Celebrate these wins in town halls and internal newsletters: “ERP already solved this specific problem.”
Days 60–90 – Lock momentum.
Document what worked and what failed. Refine roles, permissions and CSV documentation templates. Put numbers on the table — DSO improvement, batch incident reduction, time saved for QA review — and use them to confirm C‑suite sponsorship and resource protection for the next wave.
From a Board perspective, this is where data‑driven insights become tangible: they see trend lines, not Gantt charts.
Step 5 – Build a culture that can live with ERP
Even in validated cloud ERP environments, four fears often block adoption: fear of job loss, fear of getting it wrong, workload fatigue and a lack of recognition for key users.
A CFO or COO who wants sustainable medtech team engagement has to make these explicit:
- Job loss: Clearly show how roles evolve and back it with real reskilling time. More efficient and less copy‑paste. More investigation and decision‑making.
- Getting it wrong: Provide sandboxes and peer training; guarantee that early mistakes during pilots will never be used against people.
- Workload: Negotiate backfill or temporary help; be honest with investors that you are buying capacity, not just licenses.
- Invisibility: Put ERP work into objectives, bonuses and promotion criteria; thank key users publicly.
This is where the CFO’s role changes from “project policeman” to “sponsor of progress”. Acting as translator between Board, quality, supply chain and IT, the CFO can say: “Give me your biggest pain; by next phase, we will have moved the needle and here is how we’ll measure it.”
Over time, this moves the organisation towards a more Teal‑like operating model with more self‑management, more wholeness, and a clearer sense of evolutionary purpose, without needing a big ideology shift. It simply makes ERP and business transformation in pharma feel like a series of manageable steps rather than a one‑off trauma.
Download the ultimate Digital Transformation Roadmap here.
No e-mail required.
#GOATConsultants™-Level Insights:
Executive takeaways for biotech, pharma and medtech leaders
- Treat Phase 0 as a Board decision, not a workshop series. Tie ERP explicitly to launch, compliance and scaling goals.
- Frame every decision in Cash, Risk and Growth. Use life sciences ERP systems to move specific KPIs, not generic “efficiency”.
- Select champions your people already follow. Warriors, not princes, win adoption on the shop floor.
- Stack quick wins in the first 90 days. Pilots, hard numbers and clear communication create operational resilience in biotech and pharma.
- Name the fears and own the culture. When people feel safe, recognised and developed, ERP becomes a value engine, not a dread project.
For life sciences leaders serious about operational strategy and business transformation, the question is no longer “Should we implement ERP?” but “Are we willing to run ERP like we run our science: hypothesis‑driven, measured and humane?”

Enjoy more great insights:
- Latest News On Tariffs In Life Sciences
- Trump’s Biotech Policy Is Reshaping Global Science and Pharma Supply Chains
- Tariff Exemptions in Life Sciences – A Closer Look at the April 2025 Update
- Licensing Agreements In Life Sciences Amidst Trade Wars and Regulatory Shifts
- Tariff War In Life Sciences: How to Manage Customs Risks and Costs
- Medicine Shortages in the EU: Root Causes and Strategic Solutions
Enjoy Our Success Stories:
- VAT Compliance Failures Nearly Cost Biotech Millions
- Biotech customs compliance: How we saved €800K in life-saving medicine
- VAT Compliance As An Asset: How We Turned €250K into €1.9M – 660% ROI
- You cost a Customer One Million Euro in VAT penalty
- How we cut €100K/year in VAT compliance costs
- How we recovered €800K from German VAT authorities












