
Building a Resilient Supply Chain to End Medicine Shortages in the EU

Why Medicine Shortages Persist
Medicine shortages in the EU and globally have become a significant concern, impacting patient care and the overall healthcare system. This article aims to analyze the root causes of these shortages and propose strategies to prevent and mitigate their risks. Additionally, it explores how modern Enterprise Resource Planning (ERP) systems and digitalization can enhance supply chain efficiency and resilience.
“Data-Driven Strategies for Builders & Doers in Life Sciences: The Podcast”
1. Challenges of Medicine Shortages in the EU
The European Union is currently facing significant challenges due to ongoing medicine shortages, with 33 medicines in short supply. These shortages are particularly affecting critical therapeutic areas such as Diabetes Mellitus(i) (Type 2 and general), cancer treatments, and other chronic conditions. For instance, approximately 23 million individuals with Type 2 Diabetes and with 5% of the resident population in European countries estimated to have had a cancer diagnosis in their lifetime(ii), people living with cancer are potentially impacted by these shortages.
Additionally, rare conditions like Acute Promyelocytic Leukemia and Hypoparathyroidism, as well as common issues such as tobacco use cessation and bacterial infections, are also affected. The widespread impact underscores the urgent need for effective strategies to ensure a stable and reliable supply of essential medicines, thereby safeguarding patient care and public health across the EU.
The FDA also showcases the interdependencies of global manufacturing chains, including for Active Pharmaceutical Ingredients (APIs), exemplifying that the European Union is the second source of manufacturing of APIs (31%), behind Asia (45%)(iii)+(iv).
2. Root Causes of Medicine Shortages in the EU
The root causes of medicine shortages(v) in the EU are multifactorial, involving various regulatory, manufacturing, economic, and supply chain issues. Key factors include:
2.1 Manufacturing and Quality Issues: 51%
- Quality Control Problems 40% (vi)
- Good Manufacturing Practices (GMP) Violations: Non-compliance with GMP can lead to production halts. For example, contamination or deviations from standard procedures can result in product recalls or shutdowns.
- Quality Assurance Failures: Inadequate quality assurance processes can lead to defective products reaching the market, necessitating recalls and production stoppages.
- Production Disruptions 35%(vii)
- Equipment Failures: Malfunctions or breakdowns in manufacturing equipment can halt production lines.
- Supply Chain Interruptions: Shortages of raw materials or Active Pharmaceutical Ingredients (APIs) can disrupt production schedules.
- Natural Disasters: Events like earthquakes, floods, or pandemics can impact manufacturing facilities and supply chains.
- Capacity Limitations 25%(viii)
- Insufficient Manufacturing Capacity: Limited production capacity can lead to bottlenecks, especially during periods of high demand.
- Scaling Challenges: Difficulty in scaling up production to meet sudden increases in demand can result in shortages.
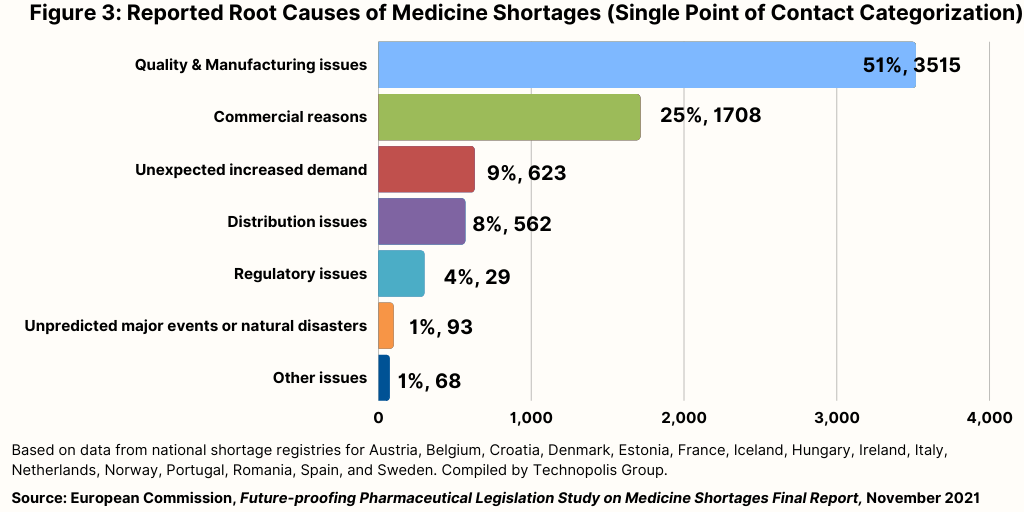
2.2 Economic Factors: 25%
- Lack of incentives to produce less profitable medicines.
- Market conditions that do not reward manufacturers for mature quality management systems
2.3 Supply Chain, Demand and Forecasting: 15%
- Bottlenecks in the supply chain, including shortages of raw materials and Active Pharmaceutical Ingredients (APIs).
- Poor public forecasting and planning-Inefficiencies in logistics and distribution.
- Unexpected surges in demand due to public health emergencies.
2.4 Regulatory Issues: 9%
- Delays in marketing authorization and regulatory approvals.
- National requirements that hinder the timely launch of products

3. Strategies to Prevent and Mitigate Medicine Shortages in the EU
To address these root causes, a comprehensive strategy involving multiple stakeholders is essential. Key strategies include:
3.1 Improved Manufacturing Practices
- Encourage investment in manufacturing capacity and quality management systems.
- Develop contingency plans to address production disruptions
3.2 Economic and Market Stability
- Create economic incentives for the production of less profitable medicines.
- Ensure fair pricing and market access systems that reflect economic and healthcare needs
3.3 Supply Chain Optimization
- Enhance transparency and cooperation among supply chain stakeholders.
- Utilize data from the European Medicines Verification System to monitor and address bottlenecks.
3.4 Emergency Preparedness and Response
- Establish a common portal for standardized reporting of shortages.
- Implement temporary emergency measures to prevent shortages due to exports.
3.5 Enhanced Regulatory Framework
- Harmonize and streamline regulatory processes across the EU to reduce approval times.
- Implement regulatory incentives for essential low-priced medicines.
4. Leveraging Modern ERPs and Digitalization to End Medicine Shortages in the EU
Modern Enterprise Resource Planning (ERP) systems and digitalization can significantly enhance quality management and minimize production disruptions in the pharmaceutical industry. Key benefits include:

4.1 Enhanced Visibility and Transparency
- Real-Time Monitoring: ERP systems provide real-time visibility into production processes, enabling early detection of potential issues.
- Traceability: Digital tools ensure complete traceability of raw materials and finished products, facilitating quick identification and resolution of quality issues.
- Improved forecasting and demand planning through advanced analytics.
- Advanced Risk Management through predictive analytics and contingency planning.
4.2 Improved Quality Control
- Automated Quality Checks: ERP systems can automate quality control checks at various stages of production, reducing the risk of human error.
- Standardized Processes: Digitalization helps standardize manufacturing processes, ensuring consistent product quality.
4.3 Predictive Maintenance
- Equipment Monitoring: IoT-enabled ERP systems can monitor equipment health and predict maintenance needs, preventing unexpected breakdowns.
- Reduced Downtime: Predictive maintenance minimizes downtime, ensuring continuous production.
4.4 Efficient Supply Chain Management(ix)
- Inventory Management for optimal inventory levels, ensuring the availability of raw materials and APIs.
- Quick Adaptation to changing market conditions and disruptions.
- Efficient Management of cross-border transactions and central stocks.
- Seamless Integration of various supply chain functions and stakeholders.
- Supplier Collaboration: Digital platforms facilitate better collaboration with suppliers, improving the reliability of supply chains.
- Reduction in operational costs through automation and process optimization.
4.5 Regulatory Compliance
- Automated Documentation: ERP systems automate the documentation required for regulatory compliance, reducing the risk of non-compliance.
- Audit Trails: Digital records provide comprehensive audit trails, simplifying regulatory inspections.


Key takeaway: Digitised processes are key to identify early deviations.
#GOATConsultants™-Level Insights:
Time to Expect More
Addressing medicine shortages in the EU requires a multifaceted approach involving regulatory reforms, improved manufacturing practices, economic incentives, and supply chain optimization. Leveraging modern ERP systems and digitalization can significantly enhance supply chain efficiency, making it more resilient and responsive to disruptions. By implementing these strategies, the EU can ensure a stable and reliable supply of medicines, ultimately improving patient care and healthcare outcomes.

Make the most of 2025 with our Key Financial Focus Articles.
- Latest News On Tariffs In Life Sciences
- Trump’s Biotech Policy Is Reshaping Global Science and Pharma Supply Chains
- Tariff Exemptions in Life Sciences – A Closer Look at the April 2025 Update
- Licensing Agreements In Life Sciences Amidst Trade Wars and Regulatory Shifts
- Tariff War In Life Sciences: How to Manage Customs Risks and Costs
- Medicine Shortages in the EU: Root Causes and Strategic Solutions
Learn more about financial management in the life sciences.
- Mastering Licensing Agreements Financial Management for Life Sciences Success

- Is Finance The Weak Link in Biotech Innovation?

Sources
- https://www.statista.com/topics/8760/diabetes-in-europe/
- https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/cancer-burden-1-20-europeans-has-faced-cancer-diagnosis-their-lifetime-2024-01-31_en
- https://www.wolftheiss.com/insights/navigating-scarcity-solutions-for-medicine-shortages-in-the-eu-central-and-eastern-europe/
- https://www.efpia.eu/media/413448/policy-proposals-to-minimise-medicine-supply-shortages-in-europe.pdf
- https://www.ema.europa.eu/en/news/ema-takes-further-steps-address-critical-shortages-medicines-eu
- https://www.dcatvci.org/features/the-eu-and-medicines-shortages-root-causes-and-mitigation/
- https://hmpi.org/2019/11/02/identifying-and-solving-the-problem-of-poor-quality-drugs/
- https://yanalytics.org/research-insights/supply-chain-disruptions-medical-goods
- https://ctl.mit.edu/sites/ctl.mit.edu/files/theses/scm2020-elgersma-capacity-and-optimization-for-pharmaceutical-industry-capstone_0.pdf
- https://flevy.com/topic/pharma/question/building-resilient-agile-pharmaceutical-supply-chain-key-factors





